
CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2 (Carbonate Ion) YouTube
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion). For the CO3 2- structure use the periodic table to find the total number of valence electrons.

SOLVED Draw the Lewis structure of CO. What is the electron geometry, molecular shape, and
I quickly take you through how to draw the Lewis Structure of CO3 2- (Carbonate Ion). I also go over the resonance, hybridization, shape and bond angle.
CO23. Solutions to Selected Problems, CO19 Chemistry LibreTexts
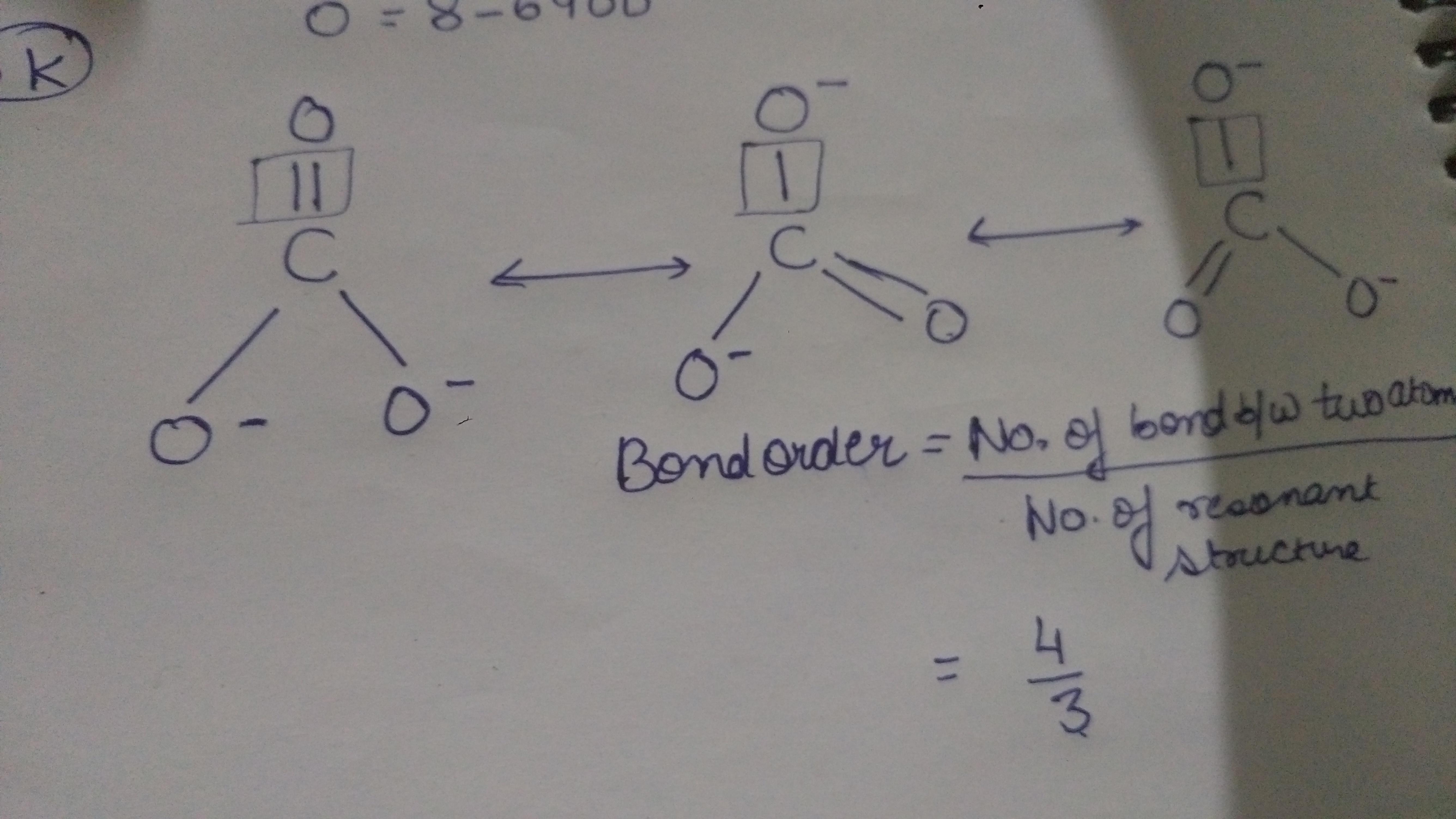
Step - 8 Last is to determine shape, hybridization and bond angle of CO32- lewis structure. CO32- lewis structure.. Carbonate (CO32-) ions have 2- negative formal charge and also it has quite sufficient lone electron pairs present on three O atoms out if which two O atoms have -1 negative charge. Thus it can easily gain or accepts H+ ions.
¿Cuál es la estructura de Lewis de Co3 2?
1. Count the total valence electrons in [CO3]2- The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [CO 3] 2- is to count the total valence electrons present in the concerned elemental atoms.
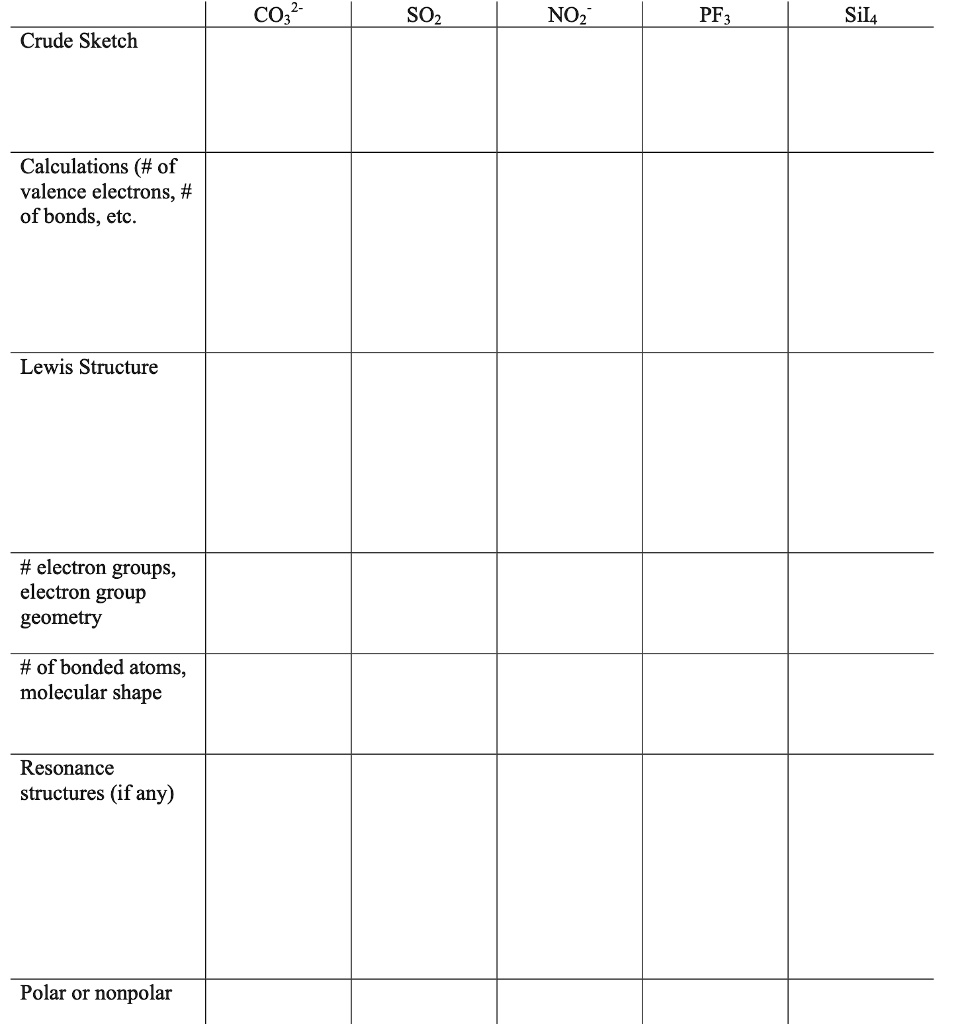
SOLVED CO3 SOz NOz PFz Sil4 Crude Sketch Calculations ( of valence electrons, of bonds, etc
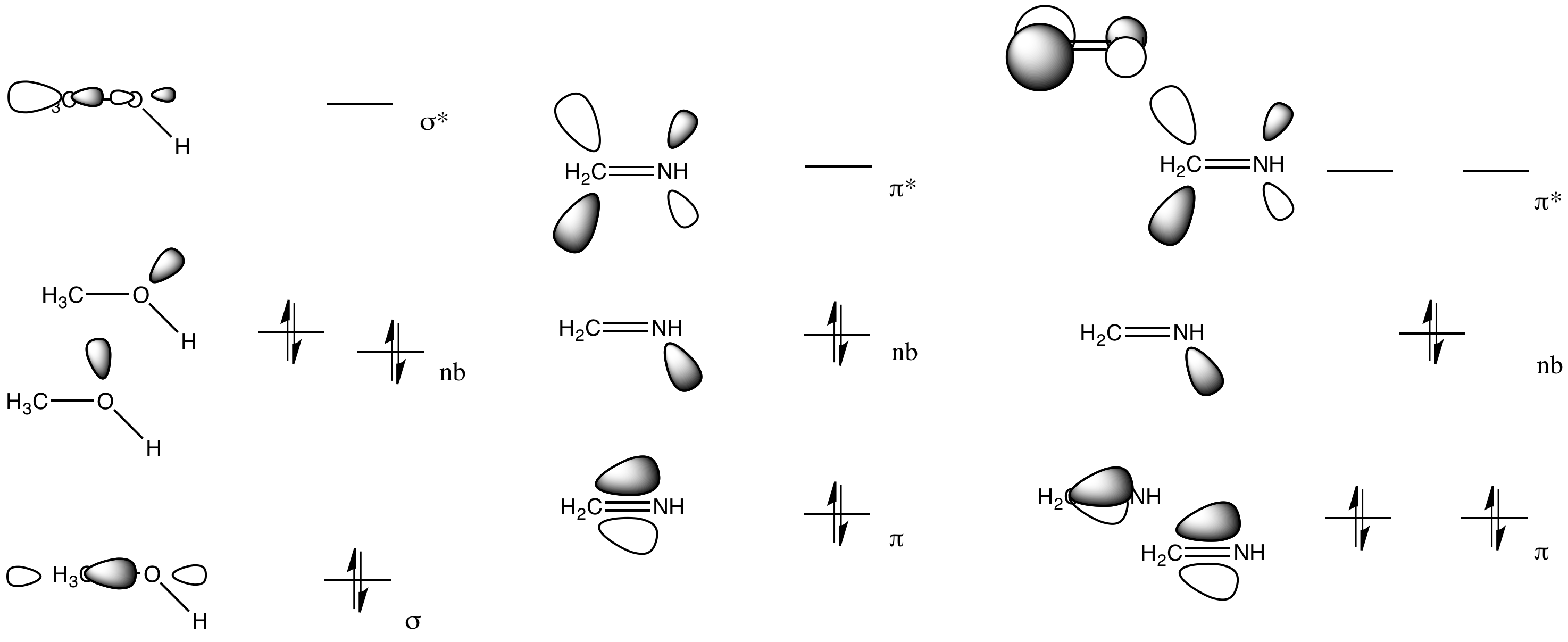
Figure 5.2.9 5.2. 9: (a) H2O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.2.3 5.2. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.

Lewis dot structure CO3 2 How to draw Lewis structures for ions Carbonate Ion Chemical
The shape of the CO3 (2-) molecule is trigonal planar, which means that the molecule is symmetrical and the bond dipoles cancel out. This results in a non-polar molecule overall, even though each bond is polar. The polar bonds cancel out each other's dipole moment due to the symmetry of the molecule, resulting in a net dipole moment of zero.

[Download 35+] Possible Resonance Structures For Co32
CO3 2- Lewis Structure Step-by-Step Guide 1. Determine the total number of valence electrons In the carbonate ion (CO3^2-), carbon (C) contributes 4 valence electrons, while each oxygen (O) atom contributes 6 valence electrons. Since there are three oxygen atoms, the total number of valence electrons is:

what is the shape of SO3 , CO3^2, NO3^1plx explain how with Lewis dot structure .. m very
10.3: VSEPR Geometry. To use the VSEPR model to predict molecular geometries. To predict whether a molecule has a dipole moment. The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons.

CO3 2 Lewis Structure & Geometry YouTube
329 Share 110K views 10 years ago A quick explanation of the molecular geometry of CO3 2- including a description of the CO3 2- bond angles..more.more A quick explanation of the.

draw the resonance structure of the following substance. dickvandykemarypoppinsbanker
1.8K 161K views 3 years ago This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structure of.

Lewis dot structure for NF3.and CO3^2_ Brainly.in
The first step in drawing the CO 3 2-Lewis structure is to determine the total number of valence electrons in the molecule. This can be calculated by multiplying the valence electrons of each atom. Carbon is located in group 14 of the periodic table and has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. In CO 3 2-, which consists of one carbon atom and.

Draw the resonance structure of 1) CO3 2 2) Benzene 3) CO2 4) O3 Pls Answer It ASAP . I need
CO32- Geometry and Hybridization - Chemistry Steps Examples Geometry CO32- Geometry and Hybridization There are 4 + 3×6 + 2 = 24 electrons. The carbon goes in the middle, and the oxygens take 6 electrons each as three lone pairs: The carbon lacks an octet, so we use a lone pair from one oxygen to make a double with it.

How to calculate bond order of co3^ 2? Brainly.in
Hello Guys!CO32- ion comprises one Carbon atom and three Oxygen atoms along with two additional electrons. In this video, we find out the molecular geometry.

How To Draw Resonance Structures Foreversalary
Carbonate, \(\ce{CO3^2-}\), is a common polyatomic ion found in various materials from eggshells to antacids. What are the electron-pair geometry and molecular structure of this polyatomic ion? Answer. The electron-pair geometry is trigonal planar and the molecular structure is trigonal planar. Due to resonance, all three C-O bonds are identical.
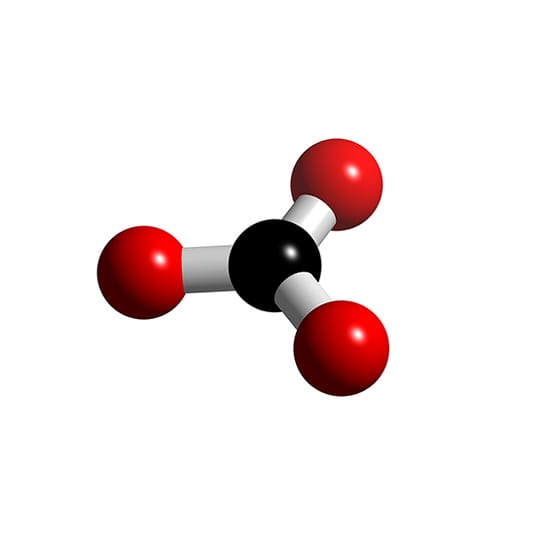
Co3 2 Molecular Geometry
Lewis structure of carbonate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of CO 32-. After finishing the lewis structure of CO 32-, there should be a -2 charge and it should be stabile structure. You will learn about these facts in this tutorial. Carbonate ion | CO 32-

What is the molecular and electron geometry of \ce{CO3^{2} Quizlet
For the CO 32- Lewis structure there are a total of 24 valence electrons available. Transcript: Let's do the CO3 2- Lewis structure: the carbonate ion. Carbon has 4 valence electrons; Oxygen has six, we have 3 Oxygens, and this negative 2 means we have an extra two valence electrons. Add that all up: 4 plus 18 plus 2: 24 valence electrons.